BG_GPCR_B1AR_reduced
About this model
This is a bond-graph model of the metabolism of the G-protein coupled receptor (beta-1 adrenergic receptor, R) and the associated Gs protein in the cardiac cell.
- INPUTS:
- Ligand (L) stimulus e.g. isoproterenol
- OUTPUTS:
- Change in molar amount of GsαGTP
- REACTIONS:
- Rswitch: Spontaneous activation of R from an inactivate state to a state that can bind a Gs protein
- LRswitch: Similar to Rswitch, but with substrate of inactive complex LR
- RL: the binding of L to R
- RC: the binding of Gs to R
- RR: the binding of Gs to complex LR
- Act1: Bundled reactions representing the transient exchange of GDP for GTP on the active RG complex, which forms GsαGTP, Gsβγ, GDP and Rtag. The latter is the R protein tagged for internalisation
- Act2: Similar to Act1, but with substrate LRG and product LRtag
- Hyd: Hydration of GTP on Gsα GTP, forming GsαGDP and phosphate
- Reassoc: Binding of GsαGDP and Gsβγ to reform Gs
- InternR: Internalisation of Rtag by the GRK and arrestin proteins
- InternLR: Similar to InternR, but for substrate LR tag
Model status
The current CellML implementation runs in OpenCOR.
Model overview
This model is based on existing kinetic model, where the mathematics are translated into the bond-graph formalism. This describes the model in energetic terms and forces adherence to the laws of thermodynamics.
For the following figures, all enzymes are shown in maroon.
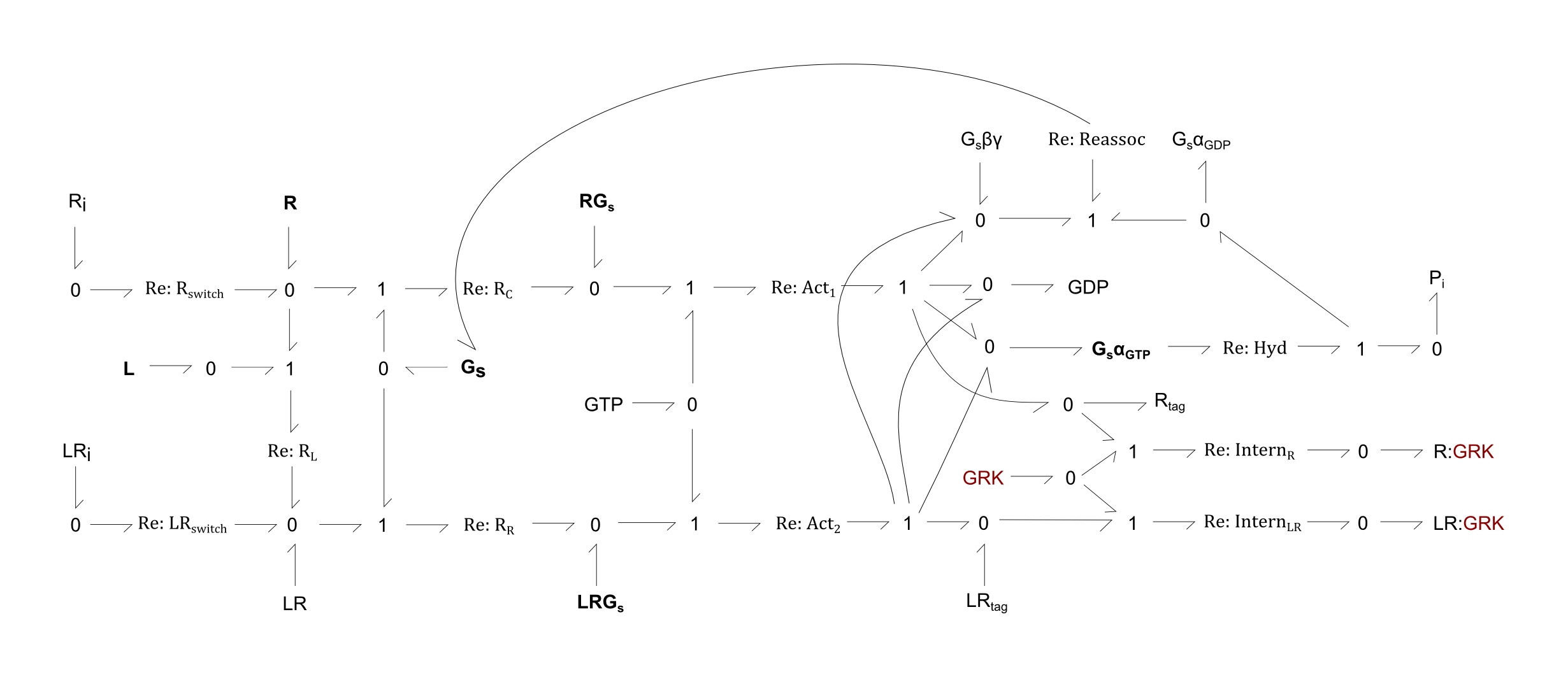
Fig. 1. Bond-graph formulation of the GPCR-β1AR network
For the above bond-graphs, a '0' node refers to a junction where all chemical potentials are the same. A '1' node refers to all fluxes being the same going in and out of the junction.
Parameter finding
A description of the process to find bond-graph parameters is shown in the folder parameter_finder, which relies on the:
- stoichiometry of system
- kinetic constants for forward/reverse reactions
- If not already, all reactions are made reversible by assigning a small value to the reverse direction.
Here, this solve process is performed in Python.
Original kinetic model
Saucerman et al: Modeling beta-adrenergic control of cardiac myocyte contractility in silico.
Additional detail on receptor internalisation were provided by Stephen Duffull et al. (University of Otago).
